Background
The pitching rate of yeast is generally accepted to be one of the most important factors in fermentation performance and the resultant beer character. The often quoted “optimal” pitching rate is 0.75 billion cells per liter of wort, per degree Plato for ale, and 1.5 billion/L-°P for lager. However, at the homebrew level, commercial yeast cultures are not available with cells counts adequate for fermentation of a typical (i.e., 20 L at 13°P) batch of ale, let alone a high-gravity lager, and multiple “smack packs” or vials of yeast can easily exceed the total cost of the remaining ingredients. The method most commonly proposed for increasing cell counts is to use a stirplate and flask, with a sponge, cotton, aluminum foil, etc. on or in the neck. There are three reasons typically given:
- Keeping the yeast in suspension mechanically increases the attenuation of wort sugars.
- Removing the toxic CO2 from solution promotes the growth of healthy yeast.
- Allowing O2 to diffuse into the starter head space maximizes yeast reproduction.
Some sources also suggest that the reduced pressure in a starter without an airlock will have a beneficial effect, but I find this claim dubious at best. The static head associated with the water in an airlock – assuming a 5 cm column – is given by:
P = ρgh = (1000 kg/m³)(9.8 m/s²)(0.05 m) = 490 Pa
which is roughly 0.5% of atmospheric pressure at sea level. This is well within the error of the experimental measurements, and so any effect present is not expected to be observable.
Experimental Method
A total of five starters were fermented out, and the volume of the resultant yeast slurry measured:
A: A control starter, with airlock.
B: A control starter, with foil wrapped over the neck in place of an airlock.
C: An airlock starter, which was swirled frequently to simulate a stirplate.
D: A foil starter, which was also swirled.
E: A foil starter, which had air added via an aquarium pump and airstone, as often as foaming would allow.
For a starter medium, ordinary granulated table sugar, which is effectively pure sucrose, was used. Sucrose was chosen not only for its low cost, but also because using DME or wort from a mash would necessarily introduce some amount of trub (hot break and cold break), which would be measured along with the slurry and could introduce some non-systemic experimental error. The use of sucrose should also allow the yeast to consume 100% of the sugars in solution, eliminating the “fermentability” of the media as a variable. Finally, using sucrose means that a refractometer can be used to take gravity measurements directly, without applying a “wort correction factor” or removing a hydrometer sample with a statistically significant volume. The starters were each made by dissolving 200 grams of sugar into 2 liters of water, which was then boiled for 5 minutes. The resulting starter solutions averaged 10.7 Brix. In order to provide the nutrients that would otherwise be lacking in an all-sugar starter, two packets of yeast (14 g) were boiled for 5 minutes in water, then topped off to 250 mL total volume. Prior to pitching, this suspension was shaken, and 50 mL added to each starter.
Into each starter, one 7 gram packet of Red Star bread yeast was added dry. Bread yeast was chosen primarily for its low cost; as a strain of S. cerevisiae, its performance in a starter should be essentially identical to any ale yeast. No effort was undertaken to control the fermentation temperature in the starters, other than placing them in a room with a household thermostat set for 68°F (20°C). To accelerate fermentation as much as possible, the starters were placed directly in front of a heating vent, and observed air temperatures ranged from 20.4°C to 25.3°C. While not particularly well-regulated, the fermentation environment therefore emulates one which would be typical for a homebrewer. After fermentation was complete, as determined by identical refractometer readings on two consecutive days, the starters were placed in a cooler at 0°C for at least 24 hours, after which the majority of the liquid was poured off and a final gravity measured via hydrometer. The slurry was then resuspended and poured into a 250 mL graduated cylinder, and the jug rinsed once with tap water. The slurry was returned to the cooler and allowed to settle for another 72 hours before measurement. This does not provide perfect isolation of dense slurry, but again, in the absence of more sophisticated equipment (a centrifuge, e.g.) it emulates the methods available to a typical homebrewer.
Finally, the slurry volumes were converted into approximate cell counts by assuming 100% viability and a cell density of 3.8 billion/mL. This inevitably introduces a great deal of uncertainty, but true cell counts are not achievable without a cytometer. From a practical perspective, a variation in pitching rate of 20%, or even more, is probably of negligible importance in brewing.
Observations
As I had only two one-gallon glass jugs (my normal starter vessels) available, the experiment was conducted in three stages. The controls, Starters A and B, were fermented first. Some variations were immediately apparent. The krausen at the surface of Starter A consisted of a thick, uniform layer of large bubbles, whereas Starter B displayed a patchier covering of comparatively fine bubbles. Visible fermentation was completed more quickly in A, with krausen having dissipated completely after 44 hours, although the airlock continued to bubble until about eight days after pitching. Both A and B also had bubbles of what I assume is CO2 coming out of solution on the glass, indicative of a supersaturated solution. In B these bubbles disappeared at roughly the same time as the krausen; in A they persisted for about six days.
Starters C and D were fermented next. To simulate the effects of a stirplate, these starters were agitated in a vigorous circular motion for approximately 15 seconds, approximately every 15 minutes, 12-18 hours a day. Clearly this is not a perfect analog for a stirplate starter, but it was observed to be sufficient to keep yeast from collecting in the bottom of the glass jug between periods of agitation. Again, marked differences in the surface appearance of the starters were apparent. Within a few minutes of swirling, bubbles began to appear at the surface of D (the foil starter); C remained clear between periods of agitation, although airlock activity resumed quickly. When swirled, C also produced fewer and coarser bubbles than D. After being measured, the slurry from D was inadvertently left in the graduated cylinder for an additional 12 days; the value of 3.8 billion/mL was obtained by assuming this allowed for full compaction to 4.5 billion/mL.
Finally, starter E was aerated using an “Elite 800” model aquarium air pump and a plastic aquarium air stone. The pump is rated for 2.0 W and 2.5 psi. Two 0.45 micron syringe-type filters were used in series to ensure sterility. The starter was aerated continuously for eight hours after pitching, and thereafter for approximately one minute in fifteen, 12-18 hours a day. This was the maximum duty cycle that was possible without the vessel overflowing. Qualitatively, this seems to be much less foam than would be expected from a wort starter of similar volume and gravity. This is sensible, given that the sucrose medium lacks most of the proteins associated with malt-based wort.
Results
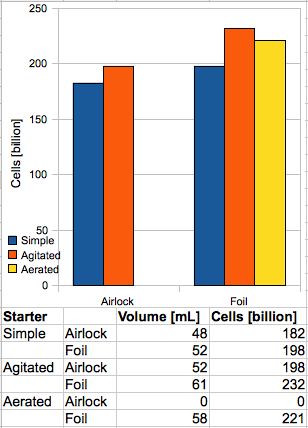
Starter B produced approximately 8% more yeast than A. Although a rigorous calculation of the experimental error was not conducted, it is almost certainly within the error bar of the graduated cylinder measurements (±0.9 mL), and so the results for the control starters offer significant support for the hypothesis that access to atmospheric oxygen increases cell growth in a yeast culture. At least part of the increase, however, can be attributed to an overall more thorough fermentation in B, which exhibited approximately 2% greater attenuation than A.
Interestingly, although the “stirplate” starters did produce significantly larger volumes of slurry, they were not nearly as large as other sources suggest. For example, MB Raines observed a four-fold increase over a starter which was shaken. The Mr. Malty™ calculator seems to assume a factor of 1.27 (for shaking) or 1.53 (for a stirplate) versus a “simple starter”. The experimental results, however, show an increase of 17% for the foil starters, and only 8% when using an airlock. It is also worth noting that C and D exhibited identical attenuation, which was statistically equal to the attenuation of A.
Finally, starter E, the aerated sample, produced slightly less slurry than D (about 5% less), which is still 12% more than the “simple starter”. Again, however, the increase is not in line with others’ results; Raines and Zainasheff report increases of 50% and 35%, respectively. The final refractometer reading for E was 0.3 °Bx higher than any other starter; I hypothesize that this is due to the liquid being saturated with air, and the fact that the hydrometer-measured gravity was also 0.5 “points” higher than the other starters would seem to bear this out. The attenuation of E was, however, roughly in line with starters A, C, and D – meaning B exhibited significantly higher attenuation than the other four samples. The reason for this discrepancy is unknown.
Conclusions
- All other things being equal, a starter covered in foil will grow more yeast than one with an airlock.
- The primary reason to use a stirplate is not the mechanical mixing of the starter, but the introduction of oxygen. Using an airlock significantly reduces the effectiveness of a stirplate.
- Contrary to what other sources indicate, a stirplate does not produce several times as much yeast per unit volume.
- Given that it can also be used to aerate the main batch of wort, an aquarium pump is probably a more cost-effective investment for a homebrewer than a stirplate.
- In the case of a pure sucrose fermentation, refractometer estimates of final gravity correlate well with hydrometer readings, with a maximum discrepancy of 1.5 “points”.
- Bread yeast (at least this brand) tends not to flocculate, and on that basis alone would be a poor choice for beer.
- I feel sorry for 17 year olds. This stuff isn’t terrible, but I wouldn’t want to drink it.
Update: 06 Feb 2010
To try to determine how much impact using sugar in place of malt would have on the behavior of a starter, I made up a 2 L starter, using 215 g of DME. In all other respects it was treated the same as starter D. Call it F. F produced 88 mL of slurry. Assuming 5% of the volume is non-yeast solids, this equates to 318 billion cells. Adjusted for attenuation, that’s 93% more yeast per unit of sugar compared to the sucrose starter. Significantly, it is also essentially identical to the figure (314 billion) given by the MrMalty calculator, when set to “intermittent shaking”. However, for a stirplate starter the calculator predicts a total of 379 billion cells. From this I draw a few additional conclusions:
- Access to free amino nitrogen can be a limiting factor in fermentations which are largely non-malt based.
- Increasing cellular access to oxygen can to a limited extent compensate for low levels of FAN.
The MrMalty calculator is sufficiently accurate at predicting cell counts for brewing, but may over-estimate the effect of a stirplate.
Update: 04 Mar 2010
I fermented out one additional DME starter, this time treating it identically to the aerated starter, E. The resulting slurry measured 116 mL. Again, assuming 5% non-yeast solids, that’s 419 billion cells. Not only is this significantly more yeast – 32% more – than the agitated starter, it’s more than the MrMalty calculator predicts for any starter handling technique, including “continuous aeration”. In fact, it’s roughly the quantity needed for a 5 gallon batch of average-gravity lager. Some final conclusions:
- Given the choice between a stirplate and aeration stone, the stone will make more efficient use of starter wort.
- By aerating, homebrewers can grow substantial amounts of yeast (such as those required for lagers) without having to make inconveniently large starters.
Update: 11 May 2010
I’ve conducted another trial in this series, to test whether or not my stirplate would in fact result in more yeast than simple agitation. It turns that it did – 100 mL, or about 361 billion cells. That isn’t quite as much as the MrMalty calculator would suggest, but it’s close, and a substantial (14%) improvement over the swirled starter. Interestingly enough, though, it still isn’t as much yeast as the aerated starter. This suggests that while the stirplate is effective at turning over the wort, introducing oxygen via diffusion, it can’t quite reach the levels achieved by actual air injection. The stirplate was run at 8.00 V for 72 hours – unfortunately, I don’t have any way to measure the actual RPM of the stir bar.
One practical benefit I’m seeing from using the stirplate is that it dissipates the foam from the aeration stone, allowing the air pump to be run continuously. I’ll be measuring one more slurry to see if this results in a further increase in volume.
Download the full experimental data:
starter_experiment.ods | starter_experiment.xls


[…] (38) Popular PostsYeast Pitching Rate ExperimentWater, Water EverywhereAeration and Yeast StartersGood Beer, Easy BeerBatch 25 Tasting NotesRegulating Fermentation TemperaturesWhy Do Bad Things […]
Why didn’t you normalize the cell count by the volume of liquid?
In a sense they are normalized, since the starter volume was the same 2 L every time. If you mean normalize to, say, a 1 L starter, though, I wouldn’t want to make the assumption that the final cell count would scale linearly with volume. (In fact, I don’t believe it would.)
Very nice experiment and write up. I would love to see the real results with a stir plate instead of the simulated stir plate action.
If my plate is ever in a state of not being used I might try this myself.
Haven’t listened to the basic brewing radio podcast yet, but maybe this was addressed in it
One thing to remember that if you are using an Aeration stone in the liquid wort you are in fact agitating the wort with the rising bubbles. A picture of your aeration setup would be nice if you have one. I was thinking of buying a Stir Plate, but seeing your information, I think I’m going to use an air pump for my starters for both agitation and oxigenation. Thanks for the site and going on Basic Brewing.
Cheers
Jim
That’s a great point, Jim. The bubble column definitely helps to turn over the wort, but from what I’ve seen in my starters it doesn’t keep all the yeast off the bottom the way mechanical agitation does. In practice, when I aerate my starters I also swirl them occasionally to keep the yeast in suspension. I was careful not to do that for this experiment, in order to keep the variables isolated as much as possible.
You can see most of the aeration setup in this photo. The only things that didn’t come from the aquarium shop are the air filters, which are standard 0.45 micron syringe filters that should be available from any surplus or lab supply store. I use two in series because 0.3 microns is generally the standard for air sanitation (a HEPA filter). The stones I use are plastic “fine bubblers” like these. I like to use them because they’re so cheap – about 50 cents – that I don’t feel guilty discarding one every few batches. That way I don’t have to be too concerned with keeping all the microscopic pores sanitary.
I appreciate the work you have done, but do not share your conclusions. A yeast starter is not a brewer’s end product.
I have done many starters, including simultaneously continuous airation, stirplate and temperature control. And all steps in between.
I find that continuously airated starters produce a lot of yeast, but are very poor beer fermenters. If you want your fermentation to stop prematurely, do continuous airation.
I have gone back to a starter on a stirplate, with an airlock and my fermentations go like crazy again. The best beers are not created with all variables optimized. When we do, we get commercial beers and those are better bought.
Interesting observation. All I can really say is that it doesn’t match my own experience. I aerate all my starters and fermentation proceeds normally, with attenuations in the 55-75% range, depending on recipe.
I don’t want to say anything bad about your beers, I’m sure they are tasty, but 55% attenuation seems pretty bad. I get 75% as an average, and usually only go lower if the yeast strain normally gets lower. I have got 80%+ just from Wyeast activator packs. I do sometimes make starters, of which I don’t aerate constantly. I just areate when I pitch and put plastic wrap over the top with a loose rubber band.
It seems that EBC might be correct, 55% seems like a fermentaiton that didn’t finish properly. Perhaps the starter yeast got accustom to an environment with abundant O2. After they go through the adaptive phase they get pooped and the change in environment stuns the productivity. From what I understand, there are two types of process required for yeast to make beer. Adaptive and Attenuative. During the adaptive phase, not much of the wort is fermented, but rather the yeast are using the O2 to replicate. These yeast have a thin cell wall so they can divide. Are these the same yeast that do the bulk of the fermentation? Once the O2 is gone, and the attenuative phase begins, the environment for the yeast changes, and they eat the sugars. Perhaps the yeat from an overly-aerated starter is spoiled with the amount of O2 they are used to and get pooped when that variable is gone.
It would be an interesting experiment if you went the extra step and used these starters to ferment identical batches of beer. This way you don’t even need to worry about proper cell count, you can instead just measure the attenuation. After all, isn’t that what we’re after?
My apologies; I should have been more clear. 55-75% attenuation corresponds to about 68-93% apparent attenuation.
I believe I was referring to absolute attenuation. When I figure my FG, I take into account the error from the alcohol present. This would be actual attenuation, correct? If that is the case, is 55% normal? I stand by my average of 75%.
If you’re compensating for the alcohol, then yes, you’re calculating the real attenuation. 75% would be very high for anything except maybe light lagers – that would correspond to about 93% apparent attenuation, or a 1.050 beer attenuating down to 1.004.
75% apparent attenuation is probably a realistic average for ales (1.050 OG, 1.012 FG), but that’s only 61% real attenuation.
Because yeast density in the sediment of the cylinder is not uniform, how can one estimate cell count based on the linear scale of the cylinder? To compare your cell counts using this unvalidated method to cell counts of others (Mr. Malty), who actually performed cell counts, is not justified. You stated that you did not make a rigorous calculation of the experimental error. In fact, you cannot make a calculation of the experimental error since only a single experiment for each variable was performed. We also don’t know that any of the values of the outcomes are statistically significantly different from each other value when only a single measurement of cell slurry volume was performed for each experimental variable. Acknowledging that this was a crude estimate of cell count, it is not appropriate to attempt to calculate the relative differences in the outcome from your different experiments.
I don’t think you can make any relevant conclusions using only sugar and boiled yeast as the medium, and it is not appropriate for you to conclude anything about the effect of a stir plate compared to what others have concluded about using stir plates (Conclusion #3 from main experiment and Conclusion #3 from 06Feb2010 update) when you used a different medium than used by others and you didn’t use a stir plate as used by others. For the same reason, I do not believe you have grounds to make Conclusion 4 from the main experiment.
You raise several good points, and I’ll address them as best I can:
I would question your assertion that yeast density is not uniform – did you mean from the top to the bottom of the yeast column, or from one trial to the next? While the density is certainly not constant throughout the column, I see no reason to believe it isn’t constant between trials. After all, three of the samples were allowed to compact fully, and the resulting rate was used to estimate the intermediate slurry density. So internal comparisons are intrinsically valid. These are the same slurry densities given by Jamil Zainasheff as well, so while they almost certainly aren’t as accurate as a cell count, they should allow for comparison to the MrMalty results, with some associated hand-waving.
You’re correct that a statistical analysis cannot be done on a single data point; I was referring to an error propagation, which most certainly can. My reasoning behind not doing one is that I was only reading the graduated cylinder to ±0.5 mL (about 1%) anyway, and that is outside the error bar of the mass and volume measurements for the starters.
Nowhere in the experiment do I directly compare the slurry volumes from a sugar starter to a wort starter – that would be an apples and oranges thing. My contention is that MB Raines’ assertion that a stir plate results in a four-fold increase in yeast volume is almost certainly incorrect, and my data suggest that the MrMalty assumption of a 27% increase may over-estimate the effect as well. As far as cost-effectiveness, even a home-made stir plate would cost roughly twice as much as an aeration setup, and regardless of its utility in propagating yeast, would be a single-use item, whereas an air pump can also be used to aerate wort in the fermenter. I stand by both statements.
I did a little light searching and found the following reference of a BYO magazine article. The reference comes from http://www.thebrewingnetwork.com/forum/viewtopic.php?p=73010
“Both practical brewers and brewing scientists have observed that yeast can be damaged when excessive amounts of oxygen are delivered during propagation. The term used to describe this stress is “oxidative damage.” While oxygen is required for a wide array of biochemical functions, it is also related to cellular aging. The free radical theory suggests that cellular aging results from damage caused by reactive oxygen species known as “free radicals” — sounds like a punk rock band!
Veronique Martin of Oxford Brookes University presented a poster at the 1999 European Brewing Congress (EBC) in Cannes entitled “The Oxidative Stress Response of Ale and Lager Yeast Strains.” This poster showed stationary phase yeast (the phase after the increase in yeast density) to be less sensitive to oxidative stress than cells growing during the exponential growth phase. Furthermore, the negative affects of oxidative stress show up in subsequent fermentations that use yeast cropped from a stressed environment. ”
I know that the aeration they are talking about is in the fermenter, not the starter, but propagation is propagation.
Also, I found this website from the basic brewing podcast episode from last week, and I also remember hearing a good idea that one listener had. He saved his yeast from the end of a white labs tube, or wyeast smack pack, after pitching rather than from the fermented batch. Perhaps taking this yeast is better for making starters as it hasn’t gone through any stress, making sure not to add to the oxygen stress.
I tend to really oxygenate my wort before/after pitching my yeast (of which if it is a starter, was not aerated to any great extent), and tend not to think about the correlation between aerating my starter, and wort. Perhaps it is too much to give it on both the starter and in the fermenter.
I wonder if any scientific work has been done to see if propagation done in the fermenter turns out better than in starters. Perhaps letting it divide and create new generations of yeast cells as the environment in the fermenter is changing is good for the yeast cells that reamin to ferment the beer. Of course, the end result of propagation has to be the same, so what you pitch in the one with the starter (without additional aeration) has to be the same as what you get from the propagation of the one you are propagating in the fermenter (with additional aeration).
I appreciate the experiments you’ve done, it is always nice for someone to address these questions scientifically. I believe the major points you came across are correct, after all, you’re experiment is on cell propagation, not beer fermentability.
I think the Mr. Wizard column you’re referencing deals primarily with pure oxygen infusion rather than aeration. The saturation point for O2 in water is about 8 ppm at STP, but using oxygen it’s possible to super-saturate the wort. At that point you may run the risk of oxidizing the cell walls themselves, but my understanding is that the risk is minimal at normal O2 concentrations. For example, the commercial yeast libraries are grown up by using continuous aeration (ref. Clayton Cone), although they also propagate below 1.002 SG to avoid the Crabtree Effect.
In my own brewery, I have several yeast strains that have been propagated for multiple generations from aerated starters, and still provide excellent (and more to the point, consistent) fermentation performance. I also believe that it’s a good idea to minimize stress by propagating yeast from the starter rather than the fermenter, and that’s actually my normal practice. I’m going to be posting a full writeup of my yeast handling procedures in the near future, as soon as I have a chance to take some better photographs.
Regarding the method of estimating yeast cell counts from the volume of the sediment, I was referring to the variable density of cells within the column of the sediment. Certainly the cell density is higher at the bottom of the column than at the top, and I’ve not seen data to confirm that the average cell count per milliliter is the same for various volumes of semi-packed cells. WIthout validation of the method, one cannot reliably compare the measured values from one experiment to another in a strictly mathematical way.
Three of the slurries (one sucrose, two DME) were allowed to compact fully, and I calculated the intermediate density by assuming that the fully compacted columns would have the density of 4.5 billion/mL cited by Jamil. All three, on a volumetric basis, compacted by the same amount (plus or minus 1 mL).
[…] This post was mentioned on Twitter by Andy Murphy. Andy Murphy said: Not using an airlock after reading this starter experiment – http://seanterrill.com/2010/01/14/aeration-and-yeast-starters/ […]
[…] (39) Popular PostsAeration and Yeast StartersYeast Ranching and YouWater, Water EverywhereGood Beer, Easy BeerYeast Pitching Rate ExperimentBM2K9 […]
[…] http://seanterrill.com/2010/01/14/aeration-and-yeast-starters/ I have some problems with the methodology, but more importantly according to the write up the stir plate version (see updates, the original experiment lacked a stir plate) resulted in substatially more yeast than the aerated version. The opposite of the claim in the OP. Does Sean Terril say different things in the podcast than he does on his blog? __________________ http://remilard.mybrute.com […]
[…] shaking is almost as good as a stirplate, and that direct aeration is the best technique of all: Aeration and Yeast Starters Overall it seems that agitation doesn't do much in and of itself (for a low-gravity wort, anyway). […]
[…] I don't know if you've seen this; it's come up in a few other threads. Aeration and Yeast Starters Nowhere near a 4x increase, but clearly agitation/aeration works. __________________ Beer is […]
Thanks Sean, for the information you provide here and on basic brewing radio. The idea of propagating yeast from a starter instead of harvesting from the fermenter seems to me an excellent one. Care to share your methods for doing this?
Another question: Those plastic aeration stones – do you sanitize them before using, if so, how?
Jerry,
I did write up a separate post on yeast propagation: Yeast Ranching and You. Let me know if there’s anything that isn’t covered there.
All I do to sanitize the plastic aerators is soak them in iodophor for an hour or so. Although to be completely honest, when it’s a new one straight from the package I don’t generally bother. I do make sure that the air pump is running the entire time the stone is in contact with the wort. Hopefully that means that little to no wort will end up inside anyway. And after using a stone two or three times I just throw it away.
Sean
[…] than I’m willing to make at this point. Keep in mind that the effect of a stir plate can be approximated by simply swirling your starter for 10 or 15 seconds every 15 minutes for the 12 to 24 hours that your starter is […]
Why do you suppose that the air-locked starter that was air-injected had zero yield? It can’t be from lack of O2. Unless… Perhaps that air-lock presented enough resistance to the air pump-air stone situation that no air was actually pumped into solution. Did you observe any bubbles? That’s a really curious anamoly that doesn’t seem to fit with the rest of the data.
You’ve established yields with DME with aerated and stirred starters. Now, how about the next step: both continous aeration and stirring together. If you need to control foaming, a drop of Fermcap S should do the trick. Sometimes synergy has interesting effects. Besides, if you’re a homebrewer taking the trouble to do the one, you might just as well do both, right?
In spite of the microbiolab types decrying the ‘unscientificness’ of your lack of high end microbio equipment to perform CFU’s and such, this is a very nice write-up with some astute observations. Nice job. It often appears to me that access to high end microbio equipment is inversely proportional to the motivation to perform these kind of experiments for the good of all. And that’s fine. I just wish they’d support their criticisms of experiments like this with some detailed data of their own. Or maybe mail you the equipment that you so obviously would put to use for the good of all. Or just shutup.
Hey Dave, thanks for the feedback. Sorry the chart is misleading; there was no aerated starter using an airlock, simply because I couldn’t find a two-hole stopper that fit a 1 gal jug.
Kai Troester has done some great experiments on yeast growth in starters. He found that aerating and stirring does increase cell counts [1], but also that the “over-aerated” yeast doesn’t attenuate as well in the primary fermentation [2]. It may be that increased access to oxygen in the starter reduces the yeast’s ability to ferment anaerobically. I’m hoping he follows up with more trials.
Cheers,
Sean
[…] is also data from an experiment you can read about here that suggests aeration via aquarium pump or agitation, plus leaving the starter unsealed to the air […]
Did you ever run one more experiment using continuous aeration with the stiplate running to see what growth you would get?
Not as an experiment, but that’s how I propagate yeast normally and counts range around 120-150 billion per liter of starter wort.
Is there a yeast calculator for continuously aerated starters as opposed to stirred starters? I continuously aearate using an aquarium filter without a stone to avoid frothing and “seem” to get more yeast than what yeastcalc using Kai’s stirplate option predicts. I say seem because all I’m really doing is looking at the yeast sediment and comparing the volume of yeast sediment to my experience with intermittent shaking using larger starter volumes to produce an appropriate amount of yeast.
Are you aware of any research between different ways of making starter that included an ethanol analysis of the finished starter?